What Is the Frequency of This Radiation 650nm
407 x 10-19 Hz. Amphenol RF - Amphenol RF Adds New AMC to BNC Adapter to its Ultra-Miniature Product Portfolio - May 02 2022.
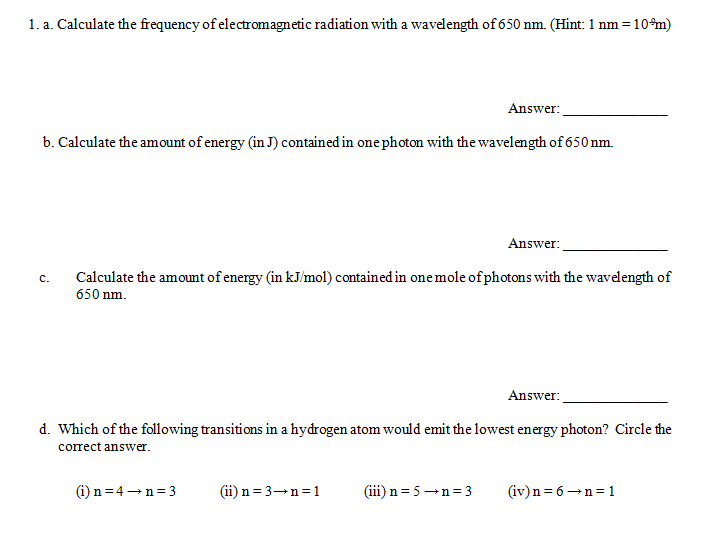
Solved Calculate The Frequency Of Electromagnetic Radiation Chegg Com
191 neV - 689 neV.
. The maximum sensitivity is usually between 500 and 550 nm. 30 MHz - 300 MHz. 180 m - 10 m.
100 mW red laser pointers probably only about 20 milliwatts and green. 124 neV - 124 μeV. Notes that 650 has only 2 SF.
C speed of light 3x108 ms λ wavelength of orange light nm First f cλ can be used to replace f in Ehf making the revised formula E hcλ. 205 x 10 6 Hz. 167 MHz - 30 MHz.
10 m - 1 m. It ranges in wavelength from approximately 400 nanometers 4 x 10 -7 m which is violet to 700 nm 7 x 10-7 m which is red. It is also known as the optical spectrum of light or the spectrum of.
Problem 621 A laser pointer used in a lecture hall emits light at 650 nm. Hence the speed frequency and wavelength of the refliegth of the reflected light are 3108 ms 51014 Hz and 589 nm respecticely. Which object is hotter and according to Wiens law how many times.
19910-26 J - 19910-25 J. Calculate the wavelength of light that has a frequency of 52 x 1012 1s. Part A What is the frequency of this radiation.
This is the answer of part A no part of me temperature. C f λ. What does radiation dose depend on.
H 6. The visible light with the wavelength of 650 nm will appear red. University of Arizona Researcher Develops New Method For Measuring Radio Antennas - May 02 2022.
A bulb is emitted electromagnetic radiation of 6 6 0 nm wave length. Red laser pointer wavelength 630-670nm green laser pointer is more for longer. 6 1 0 3 4 J s C 3 1 0 8 m s Medium.
680nm 1lm 109 nm 680 107 m. Part A What is the frequency of this radiation. 461 kHz - 167 MHz.
30610-28 J - 1110-27 J. The speed of a light wave is known as c or 299792458 meters per second and you already have the wavelength of 680 nanometers. Hence frequency of radiation is directly was not to temperature since frequency for radiation age less than be hence temperature of a is less than the top B.
What is higher as the frequency is higher in a wave. Type of radiation exposure time. Keysight Technologies - Keysight Enables Xiaomi to Validate 5G Technology in Smartphones and IoT Devices - May 02 2022.
If you convert 650nm to meters you get 65x10-7 m so you can divide using the formula above giving you 46x1014 1s or Hz the unit of frequency in Physics. What is the frequency of this radiation. 1 m - 10.
146 x 10 2 Hz. The total energy of radiation is 3 1 0 1 8 J. 689 neV - 124 neV.
UHF ultra high frequency decimeter band. The visible light spectrum is the section of the electromagnetic radiation spectrum that is visible to the human eye. The frequency f of this light EM radiation is inversely proportional to the given wavelength in meters where c is the speed of light.
The number of emitted photon will be. E n h ν 602 X 1023 photons mol 6626 X 10-34 Js. Frequency of this light.
X 10-12 m 280 x 10-10 m To observe an object we need the wavelength to be as small or smaller than the object being viewed. F is the frequency of the wave in inverse seconds s or hertz Hz c is the speed of light in vacuum precisely 299792458 ms. Nowadays the More durable transmitted red light air dust water vapor so the light path can be seen to need more power.
The speed of of light is 30x 108 ms. VHF very high frequency. Electromagnetic radiation with a wavelength of 589 nm appears as yellow light to the human eye.
Problem 621 A laser pointer used in a lecture hall emits light at 650 nm. 650 nm represents the wavelength and 3x108 ms is the speed of light. F frequency Hz of orange light at 650 nm.
What is the energy of light with a wavelength of 589 nm. The energy carried by each visible photon is between 31 eV and 18 eV respectively and the frequency is in the 750 THz to 428 THz range The sensitivity of the eye to wavelengths beyond this range drops dramatically and they are represented as black in the figure below. V s-1.
Frequency f and wavelength λ are related as. What is the energy per photon of the lowest frequency of electromagnetic radiation that can be used to observe a gold atom with a diameter of 280. That of object B peaks in the red region at 650nm.
The relationship between wavelength and frequency is λcf. What is 1nm equal to in standard form. From that you can calculate the frequency of the light wave by plugging the numbers.
Since our wavelength must be in units of m well convert from nanometers to meters knowing that 1 m 109 nm. F c λ 3 108ms 086 109m 35 1017Hz. What is the frequency of a wavelength of 589 nm.
Using Figure 64 predict the color associated with this wavelength. Up to 256 cash back A laser pointer used in a lecture hall emits light at 650 nm. Visible red light with a wavelength of 700 nm for example has a frequency of 429 x 10 14 Hz and an energy of 284 x 10 19 J per photon or 171 kJ per mole of photons remember Avogadros number 602 10 23 mol 1.
300 X 108 m s-1 1 x 109 nm ν ----- ----- 461538 X 1014 s-1 46 X 1014 s-1 650 nm 1 meter b What is the energy of 1 mole of these photons. Essentially that equates to the colors the human eye can see. 1110-27 J - 19910-26 J.
F29979245868010⁹440871261810¹⁴ Hz which is about 4408712618THz. 650 m - 180 m. Which objects give out infrared.
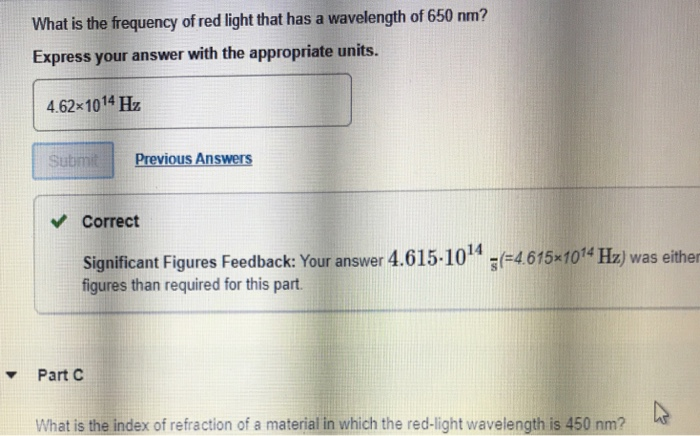
615 x 10 14 Hz. The formula for frequency is speed of light divided by wavelength. Plugging in known values to the equation and solving for f we have.
The first red lasers was released in the early 1980s. What is the frequency of this radiation. The frequency of the radiation emitted when electron falls from infinity to n1 state for he would be Kinetic energy of an electron emitted when radiation of frequency v 125 x1015 strikes the metal surface whose threshold frequency v0 725 x 1014 is Name the first five series of lines that occur in the atomic spectrum of hydrogen.
Do remember that red lasers are.

Solved What Is The Frequency Of Red Light That Has A Chegg Com
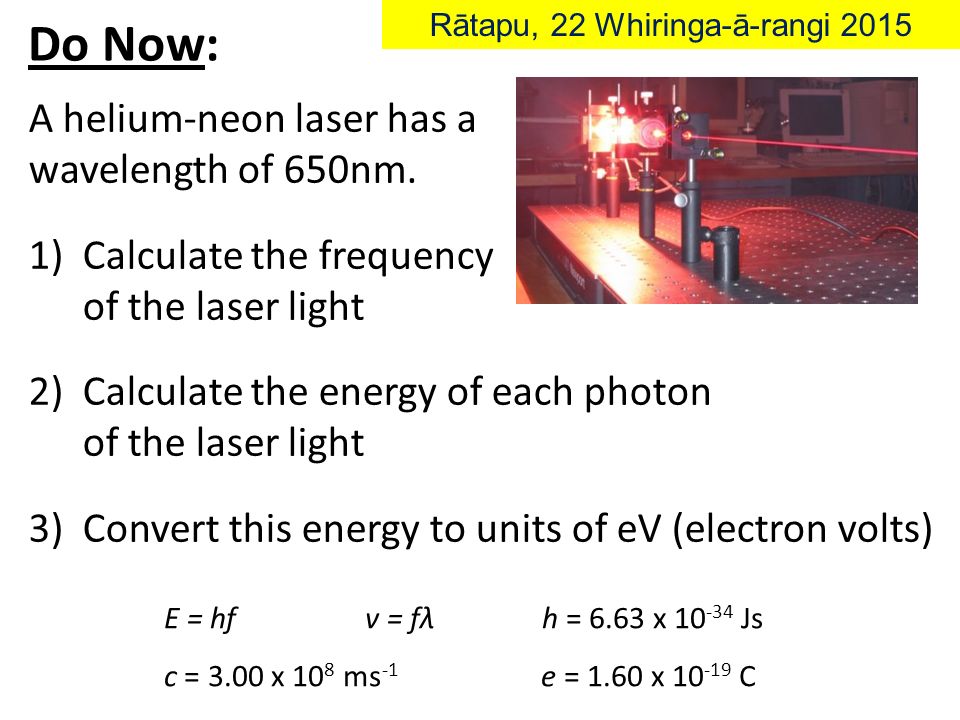
Do Now A Helium Neon Laser Has A Wavelength Of 650nm Ppt Video Online Download

Do Yellow Bug Light Bulbs Work 1000bulbs Com Blog Visible Light Electromagnetic Spectrum Visible Light Spectrum
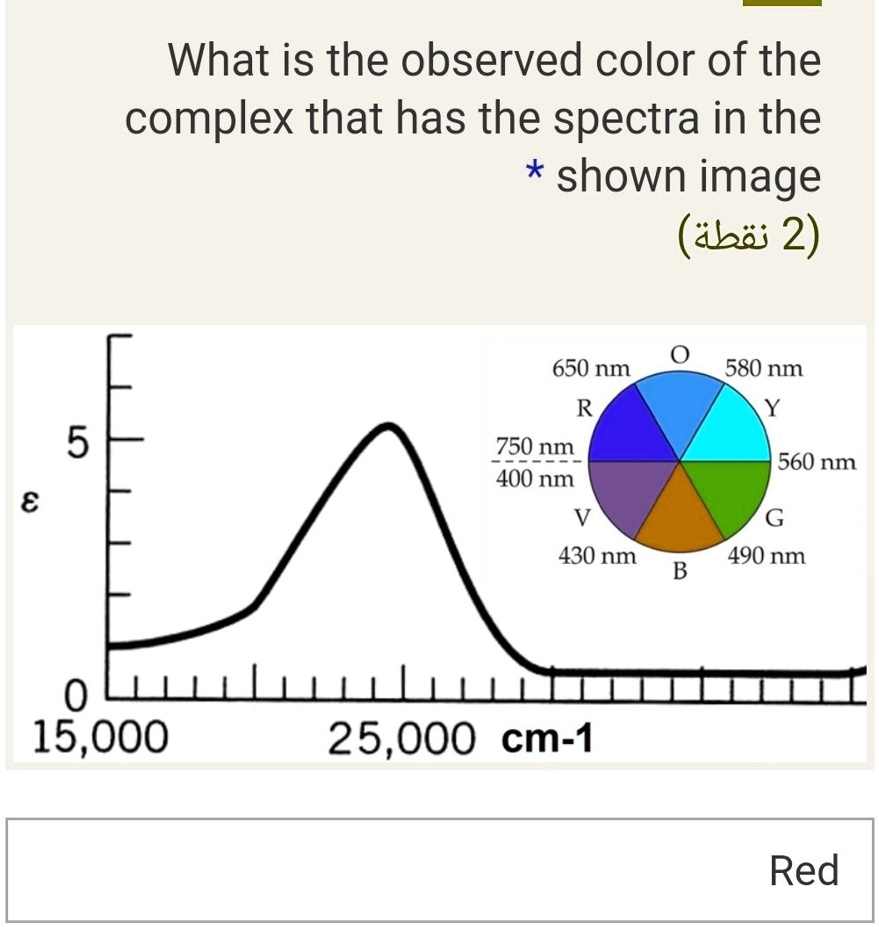
Solved What Is The Observed Color Of The Complex That Has The Spectra In The Shown Image Abii 2 650 Nm R 750 Nm 400 Nm 580 Nm 5 560 Nm 8 G 490 Nm 430 Nm B 0 Llllllllllllllc 15 000 25 000 Cm 1 Red
Comments
Post a Comment